Research and Production Sites
Life Sciences Laboratories

The Institute of Bio-Active Science and the Formulation Research Laboratories of Nippon Zoki Pharmaceutical (NZP) were integrated in 2024 at the site of the Formulation Research Laboratories. Our Neurotropin™ Headquarters and R&D Headquarters (Research Headquarters) are located within this facility.
-
Neurotropin™ Headquarters
Neurotropin™, one of NZP’s core products, is a biological formulation first developed in the 1950s as an injectable drug that is effective in treating conditions such as lower back pain, allergic rhinitis, and the after-effects of subacute myelo-optico-neuropathy (SMON) such as cold sensations, other abnormal sensations, and pain. In 1988, we developed a tablet formulation (for the treatment of lower back pain, cervico-omo-brachial syndrome, shoulder periarthritis, and osteoarthritis), with an additional indication for postherpetic neuralgia added in 1999. The results of extensive basic and clinical studies to date have shown that Neurotropin™ has unique pharmacological effects. Our Neurotropin™ Headquarters draws on the expertise of the entire organization to conduct intensive research into the essential properties of Neurotropin™. It is committed to further development of the drug, including generation of research results that will lead to the expansion of the drug’s indications in the future.
-
R&D Headquarters
Our R&D Headquarters is where NZP’s new products are born. It undertakes licensing activities aimed at introducing pharmaceutical products, medical devices, and regenerative medicine candidates from research institutions and companies in Japan and overseas, as well as research and development of in-licensed products, products developed in collaboration with academic institutions, and proprietary products. The organization oversees a restructured combination of three earlier units: research, development, and business development, and all its employees are involved in exploring research topics and drug candidates, which they seek to turn into products. The R&D Headquarters consists of our Research Division, which is based at the Life Science Laboratory, and our Development Division, which is located at our Head Office in Osaka and Tokyo Laboratory. The Research Division is comprised of departments specializing in chemistry, biology, and pharmaceutical science, while the Development Division consists of departments specializing in clinical development, statistics and data management, and business development. Meanwhile, the Tokyo Laboratory consists of departments responsible for joint research and development with other companies and academic institutions. All these departments work as one to fulfill the unmet needs of medical practitioners and deliver our unique new products to the market as swiftly as possible.
Ono Ryokuen Factory
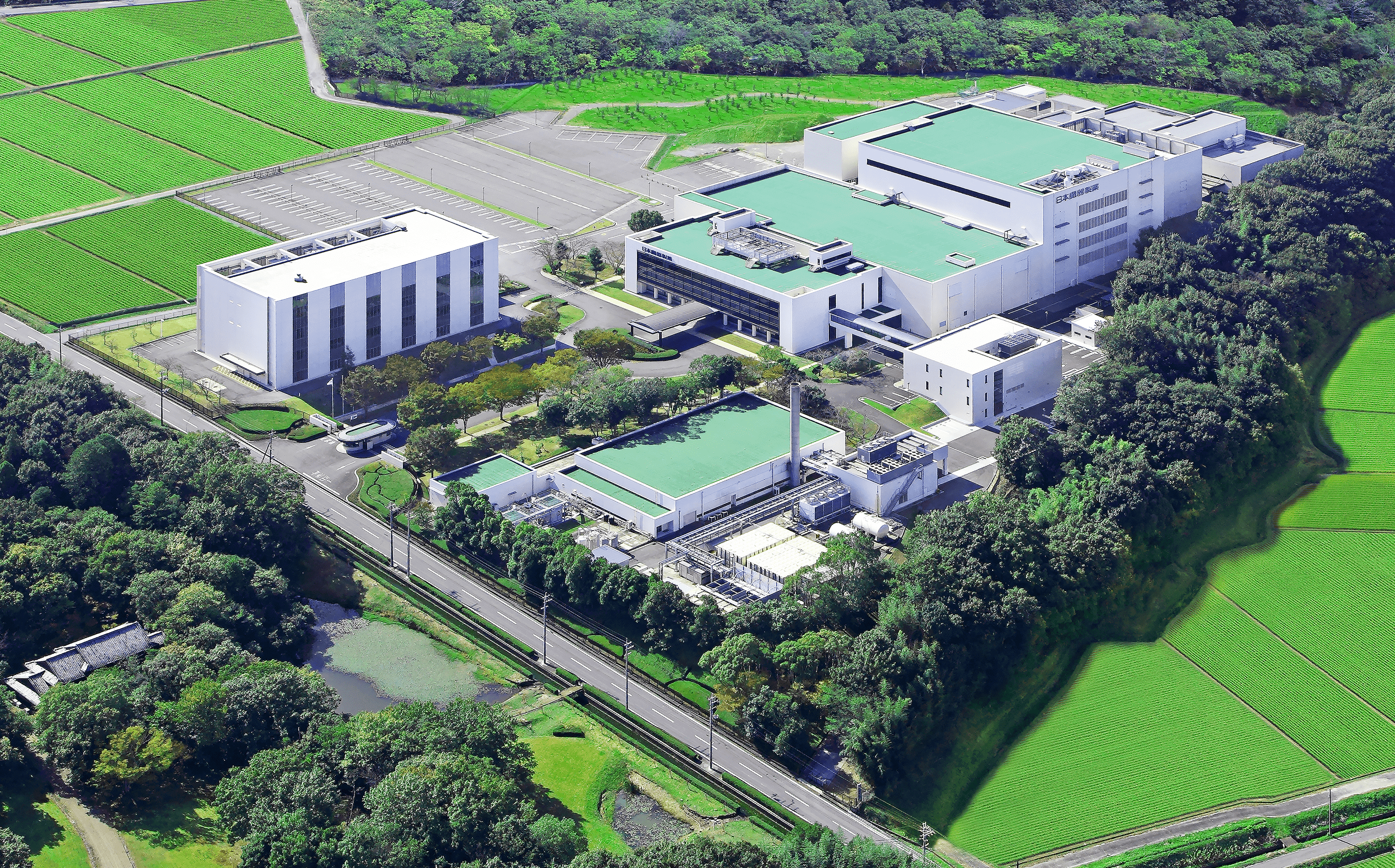
Built in 1986 on a lush hilly area in Ono City, Hyogo, Japan, the Ono Ryokuen Factory manufactures our mainstay, Neurotropin™, and other pharmaceutical products, under the most advanced production and quality controls. The 100,000 ㎡ site is lush with greenery, hence its name “Ryokuen Factory.”
-
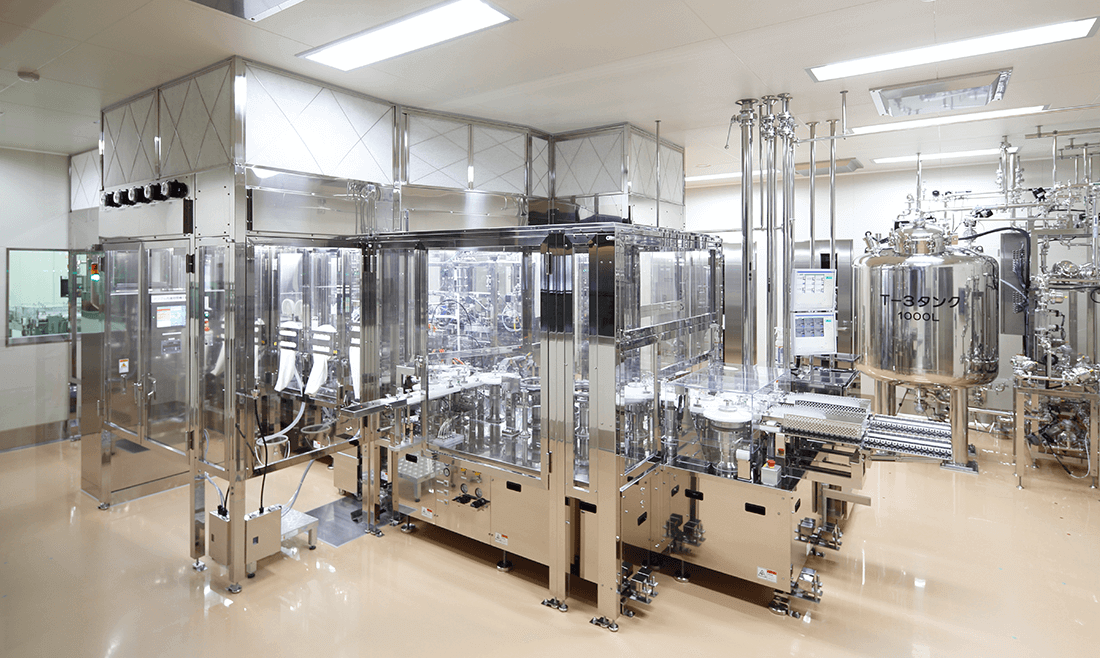
The factory conducts integrated manufacturing, from active pharmaceutical ingredients to solid preparations for oral administration or sterile injectables in compliance with Good Manufacturing Practices (GMP), an international standard for the manufacturing and quality control of pharmaceutical products. Its impeccable manufacturing system has generated high acclaim from regulatory authorities around the world. In particular, to ensure the efficacy and safety of Neurotropin™, which is made from biomaterials, manufacturing and quality controls are conducted at levels far higher than those of ordinary drugs.
-
In addition, to respond to the frequent revisions of international regulations and advances in drug manufacturing technologies, the factory proactively gathers information on the manufacturing and quality control of drugs from around the world to constantly improve the quality and safety of its products. More recently, the factory has adopted an “out-of-the-box” approach to further improve the way it works, by for example automating equipment for production and quality tests. Aiming to be a “pharmaceutical manufacturing factory that is friendly to both humans and the environment,” Ono Ryokuen Factory achieves a harmonious balance between production and environmental protection, while firmly safeguarding employees’ health. As part of these efforts, the factory conducts ongoing initiatives to conserve the surrounding environment, such as installing high-performance wastewater treatment and incineration systems.


